Author:Neil Patel, PharmD, BCPS + InpharmD™ AI
LEARN MORE

The development of IgG avidity assays has revolutionised serological diagnosis of Toxoplasma infections. The measurement of IgG avidity has shown its power in various clinical settings, especially in situations where timing and differentiation of primary and secondary infections is crucial. However, no laboratory test performed alone is self-sustained, whereby the diagnostic strategy of choice is sequential (or combinatorial) use of high-quality IgG, IgM, IgA and IgG-avidity assays. The impac...
Diagnosis of toxoplasmosis is useful for human and animal health. Several techniques are employed for the diagnosis in feline and canine population. Coprological tests for the detection of oocysts in cat faeces are of little significance owing to short patency (15 days). Histological examinations of biological samples show a lack of reliability when the animals are infected with few parasites; the mouse inoculation is the most reliable method even if the detection of cysts in mice brain require 40 days. However tachyzoites of virulent strains can be isolated from peritoneal exudate 3-4 days after inoculation. Samples inoculation in cell cultures (VERO, human fibroblasts) requires specialized laboratories and fails if non viable parasites are present due to tissutal autolysis. Serological tests are the most used diagnostic methods; Dye test and IFAT that require intact tachyzoites are more sensitive and specific compared to IHA, LA, ELISA because, during the infection, the first sign...
READ MORE→
A search of the published medical literature revealed
2 studies investigating the researchable question:
Serodiagnosis of toxoplasmosis. The impact of measurement of IgG avidity?
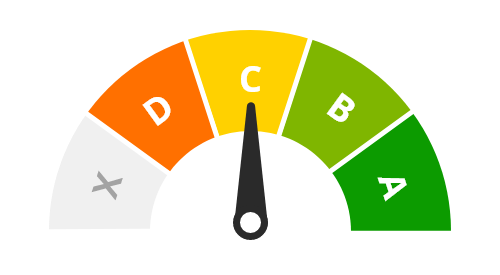
Level of evidence
C - Multiple studies with limitations or conflicting results
READ MORE→
[1] Piergili Fioretti D. Parassitologia. 2004 Jun;46(1-2):177-81.
[2] Tanyuksel M, Guney C, Araz E, Saracli MA, Doganci L. J Microbiol. 2004 Sep;42(3):211-5.
[3] Tanyuksel M, Guney C, Araz E, Saracli MA, Doganci L. J Microbiol. 2004 Sep;42(3):211-5.
[4] Liesenfeld O, Montoya JG, Kinney S, Press C, Remington JS. J Infect Dis. 2001 Apr 15;183(8):1248-53. doi: 10.1086/319672. Epub 2001 Mar 16.
Author: Open AI GPT
Serodiagnosis of Toxoplasmosis: The Impact of Measuring IgG Avidity
Introduction
Toxoplasmosis is a pervasive infectious disease caused by the protozoan parasite Toxoplasma gondii. Serological testing plays a pivotal role in its diagnosis. Among these tests, IgG avidity measurement has emerged as a vital tool in distinguishing between recent and past infections.
Understanding IgG Avidity
IgG avidity refers to the strength with which IgG antibodies bind to their antigens. During the initial stages of infection, the produced IgG antibodies usually exhibit low avidity, which gradually increases over time. Consequently, measuring IgG avidity can provide valuable information about the timing of infection.
Clinical Significance
In pregnant women, determining the timing of Toxoplasma infection is crucial due to the risk of congenital transmission. A high IgG avidity suggests an infection that occurred more than 12 to 16 weeks ago, effectively excluding recent infection and minimizing unnecessary interventions. Conversely, low IgG avidity may indicate a recent infection, warranting further monitoring and potential treatment.
Methodology
The IgG avidity test involves treating the serum sample with a protein-denaturing agent and comparing the binding of IgG antibodies to the antigen with and without the denaturant. The result is expressed as an avidity index, which helps infer the infection timeline.
Challenges and Considerations
Although IgG avidity testing is a significant advancement, it is not without limitations. Variability in test protocols and subjective interpretation can affect results. Moreover, certain populations may exhibit atypical avidity maturation kinetics, underscoring the importance of comprehensive serological assessment.
Conclusion
The measurement of IgG avidity has significantly enhanced the serodiagnosis of toxoplasmosis, providing essential insights into infection timing. When used alongside other serological tests, it enables more accurate diagnosis and informed decision-making, particularly in sensitive cases such as pregnancy.